| g e n u i n e i d e a s | ||||||
 |
 |
 |
 |
 |
 |
 |
| home | art and science |
writings | biography | food | inventions | search |
| sweating it out |
|
July 2014 It's a hazy, hot and humid morning, and you start the day already feeling half-baked. Unable to cool off. And not looking forward to the prospect of tending a stick burner for the next 14 hours... A long day ahead. But how does oven humidity affect cooking SPEED, particularly in view of the delay created by the barbecue stall? Will food cook faster? Slower? Better? Can the 14 hours drop to a more manageable 10? Instead of meat, with the added complexities of fat rendering and collagen shrinking, we chose a cellulose sponge for the experiment. An electric oven1 (fully instrumented with separate controls for the heating and fan circuits) is our outdoor "smoker". Don't worry, its not a perfect analog, but still manages to capture most of the essential physics.
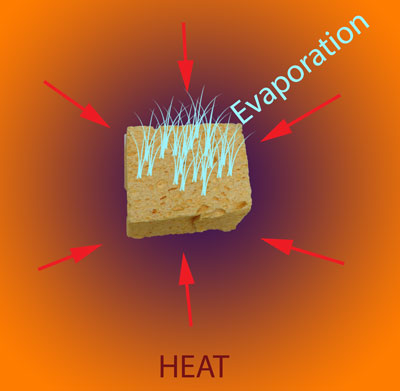
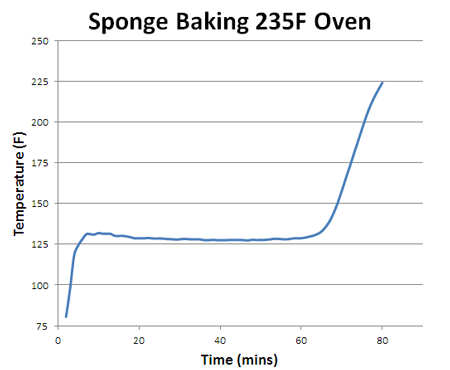
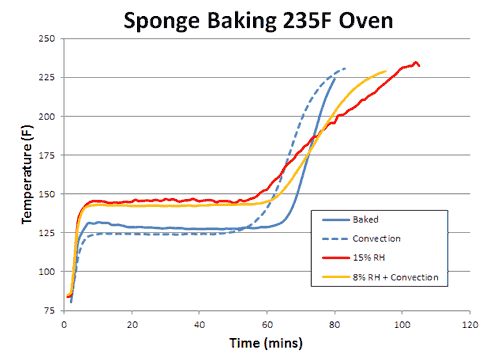
How does the sponge cook? As indicated by the red arrows, the sponge is heated from the outside in. Because the sponge (and meat) are poor thermal conductors, heat piles up at the surface, and during the initial stages of cooking, the interior lags behind. But, since the sponge is moist, it also cools off by evaporation (e.g. sweats), pushing back on the oven's heat. At some temperature the heating and cooling rates are in sync, the sponge "stalls" and stops rising in temperature. Even the air itself plays a role in how food cooks- air is a terrible thermal conductor, and if stagnant, acts like the meat is wearing a goose down coat. Blocking heat from entering, and humidity/cold from leaving. So let's start by cooking a moist sponge1 in a large electric toaster oven. As this graph indicates, the sponge quickly warms to around 125F, then stalls due to evaporative cooling2. When the flow of moisture from the interior to the exterior is nearly exhausted (at around 70 minutes) the cooling rate cannot keep up with the heating rate, and the sponge's core temperature takes off:
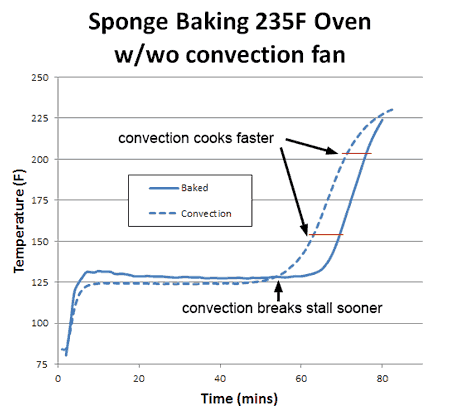
What happens if you blow away the insulating layer of air that surrounds the cooking sponge? In other words, turn on the oven's convection fan? Blowing away the insulating air has two effects. First, like sitting in front of a fan on a hot summer day, evaporative cooling increases, and the stall is now a few degrees lower in temperature (but not *that* much lower- a key observation we will return to in a moment). Secondly, the sponge exits the stall earlier, since the moist evaporating air no longer lingers around the surface where it can block subsequent evaporation. Convection does not increase the oven's temperature, but it does bring the heat closer to the meat:
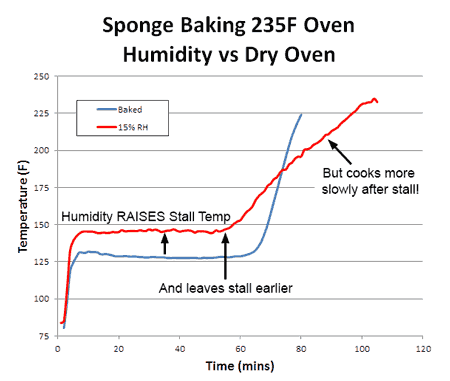
Consequently, whether you are cooking the sponge to 155F (like a pork tenderloin) or to 203F (like a brisket) the food cooks faster. With real meat, rather than a sponge, convection cooking times are often a third faster and the surface (like chicken skin) crisper and drier. What about humidity? With more humidity, evaporation is slowed (try cooling off after chopping wood on a sticky summer day) and the meat is moister. Logically, the stall should also rise in temperature. Which it does. And just as logically, one might assume if the meat is baking at a higher temperature (because the stall is at a higher temperature), it will also cook faster. But in this particular case, common sense will let you down. To understand why, we baked a sponge in 15% relative humidity- about as high as any smoker can reach with a water tray. As predicted, the stall temperature rose dramatically- by nearly 20F. The sponge also exited the stall around 20% earlier than in a dry oven. Yet final cooking times were a mixed bag:
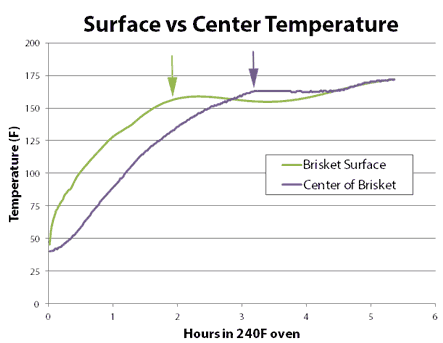
If you were cooking to 150F, the high-humidity oven gets you there sooner. But if you were cooking to 200F, the drier oven wins. Why? Heat and moisture cannot move instantaneously. Ever try wringing juice out of a slab of raw beef, or water from a damp sponge? It takes time for water to force its way out. This is the reason why the convection fan only slightly lowered the stall temperature- water could not reach the surface fast enough to evaporate and drop the temperature any farther. Cooling was limited by the rate of moisture flow, not evaporation. It also takes time for heat to diffuse inward. In this curve, we can see the large (sometimes 30F!) difference between the interior and the exterior of an actual baked brisket. The surface will quickly dry out, the surface stall end, yet there is still lots of moisture stranded in the core:
The stall allows time for the interior temperature to catch up to the exterior. When the stall ends, the temperature again begins to climb, but the remaining moisture reserve slowly leaks out into the humid air, and moderates the cooking rate. In a dry oven, with a longer and lower stall, the temperature gradient is smaller, more moisture exited during the stall, and there is less moisture available (in the air or the meat) to slow cooking after the stall. And like the tortise, eventually wins the race. Why does the Texas Crutch, where you wrap the meat in foil to completely suppress the stall, cook faster? In this case, the stall temperature is ABOVE any final cooking temperature, so you never exit the stall and the higher moisture levels in the meat play a secondary role determining cooking time. Plus 212F is much hotter than 150F. Thus a convection fan speeds up cooking by blowing away the moist insulating bubble of air which normally surrounds food. A humid oven reduces evaporation, leaving more moisture inside the food to make it to the plate. The added humidity also raises the stall temperature. Which may or may not speed up cooking....
|
|
-------------------------------------------------------------------------------------------------------- Additional articles on kitchen science can be found HERE. 1 The sponge weighed 17.6 gms wet and 8.6 gms dry. The electric oven's temperature was held to +2F by a PID controller. A digital humidity sensor monitored moisture levels 2" from the sponge. Humidity was produced by a water pan installed a half inch above the lower heating element. Porous lavastone plates were propped in the water pan to increase evaporation rates. The sponge was mounted 3" above the water pan (whose temperature had stalled around 160F) to allow space for the oven to return the humid air to 235F. Note the kitchen's relative humidity was around 60%, so the oven's humidity (without a water tray) is around 1.5%. While latent heat of condensation plays a role in high humidity cooking, with only 1 out of every 10 air molecules in the oven water, the absolute effect is small. 2 Meat stalls closer to 150F, but the sponges' enormous surface area (like a car radiator) dramatically increases its cooling efficiency, so the stall temperature drops. If interested, this chart combines all of the above results, along with adding a convection fan to a humid oven.
|
 Contact Greg Blonder by email here - Modified Genuine Ideas, LLC. |